Heat-induced Conformational Changes of Ara h 1, a Major Peanut Allergen, Do Not Affect Its Allergenic Properties - ScienceDirect

The majority of the Ara h1 IgE binding epitopes are clustered in two... | Download Scientific Diagram
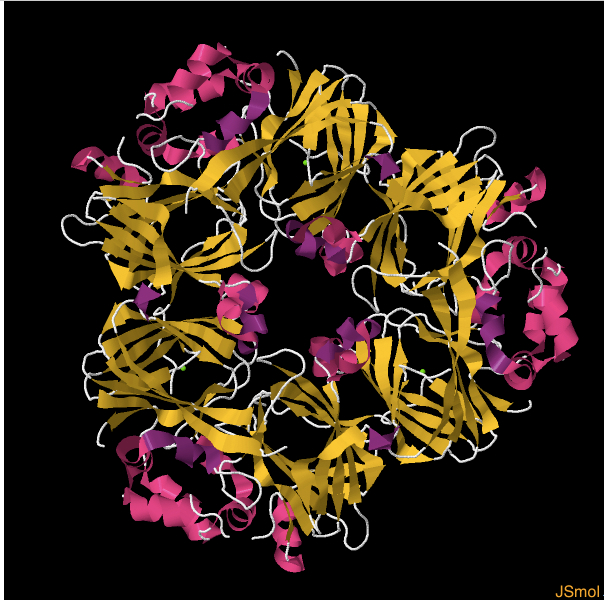
Biochemical and Structural Analysis of the IgE Binding Sites on Ara h1, an Abundant and Highly Allergenic Peanut Protein*

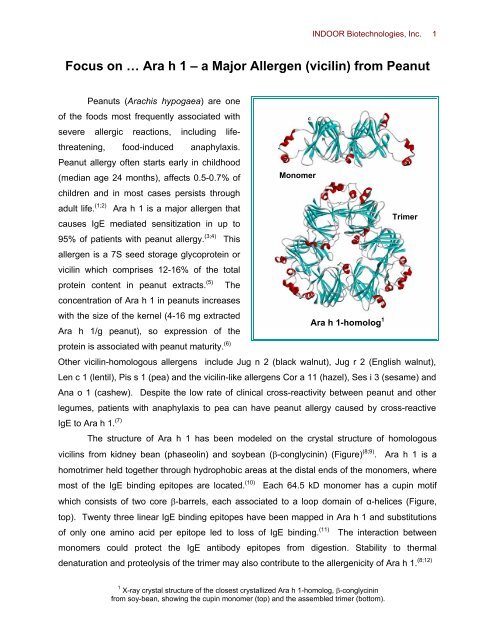
Molecular model of the Ara h 1 trimer. A space-filled, homology-based... | Download Scientific Diagram

Purification and molecular characterization of a truncated-type Ara h 1, a major peanut allergen: oligomer structure, antigenicity, and glycoform | SpringerLink

PDF) Protein structure plays a critical role in peanut allergen Ara h 2 stability and may determine immunodominant IgE binding epitopes | Moon Sen - Academia.edu

Electrochemical Detection of Peanut Allergen Ara h 1 Using a Sensitive DNA Biosensor Based on Stem–Loop Probe | Journal of Agricultural and Food Chemistry
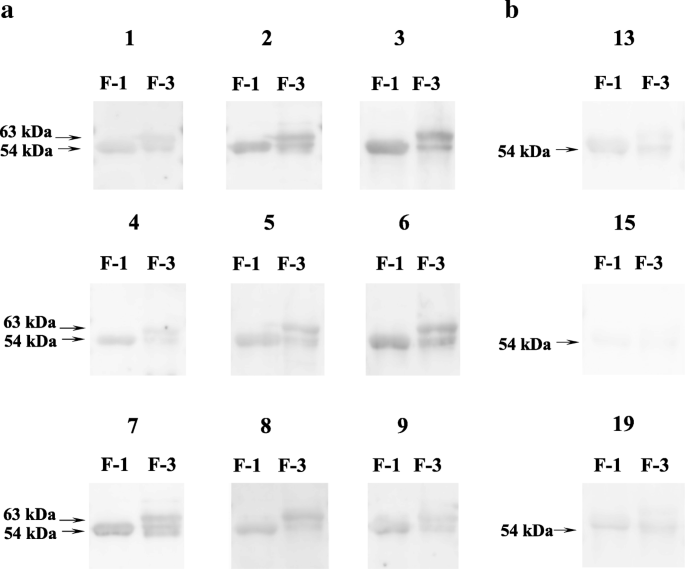
Purification and molecular characterization of a truncated-type Ara h 1, a major peanut allergen: oligomer structure, antigenicity, and glycoform | SpringerLink
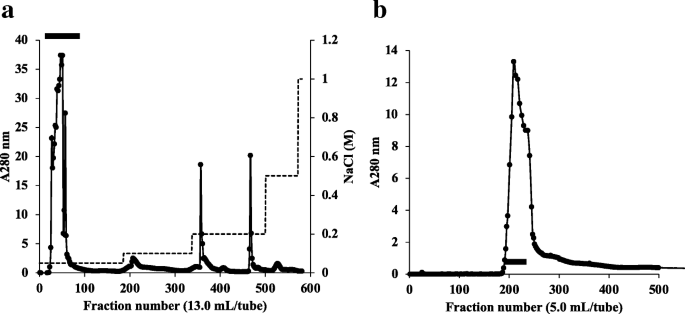
Purification and molecular characterization of a truncated-type Ara h 1, a major peanut allergen: oligomer structure, antigenicity, and glycoform | SpringerLink

Trimer formed by rsAra h 1. Ara h 1 trimer shown in three different... | Download Scientific Diagram

Ara h 6 is involved in hetero-and homopolymeric structures after peanut... | Download Scientific Diagram
Heat-induced Conformational Changes of Ara h 1, a Major Peanut Allergen, Do Not Affect Its Allergenic Properties*

Purification and molecular characterization of a truncated-type Ara h 1, a major peanut allergen: oligomer structure, antigenicity, and glycoform | SpringerLink
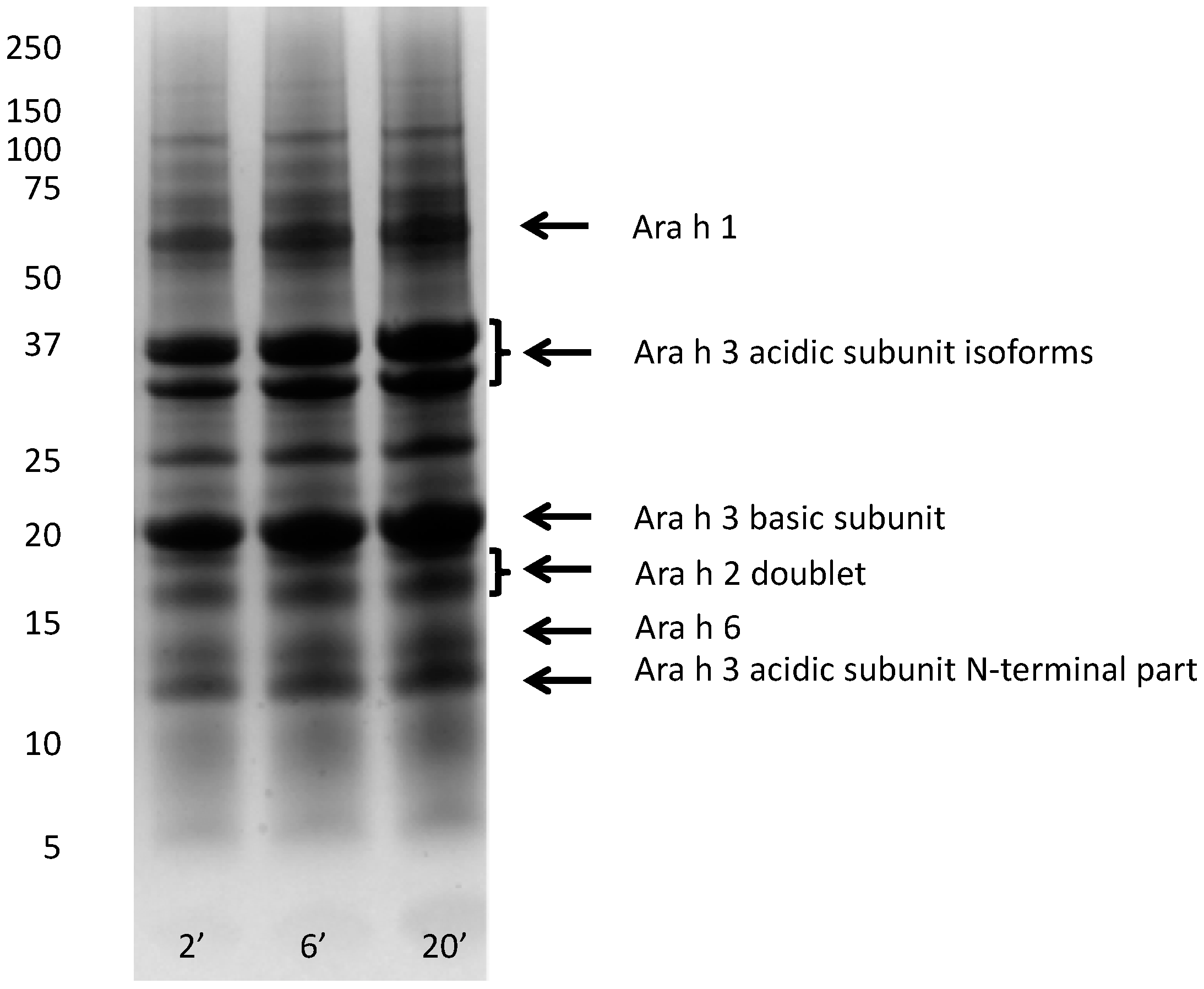
Nutrients | Free Full-Text | Release of Major Peanut Allergens from Their Matrix under Various pH and Simulated Saliva Conditions—Ara h2 and Ara h6 Are Readily Bio-Accessible