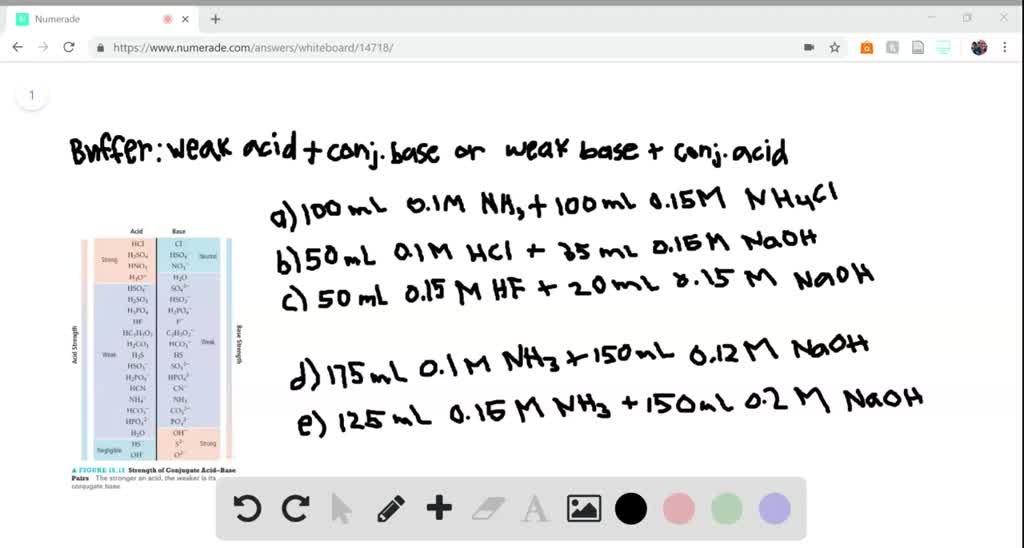
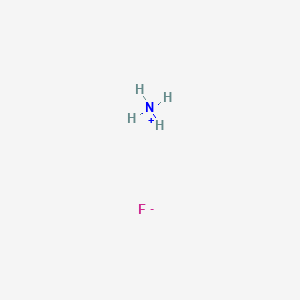
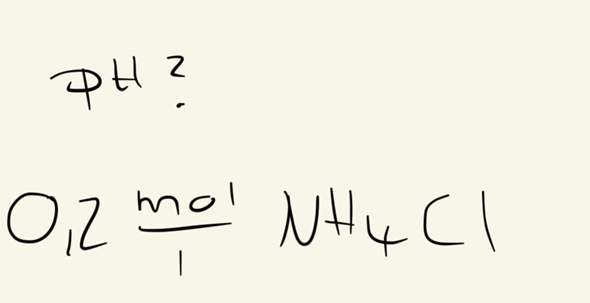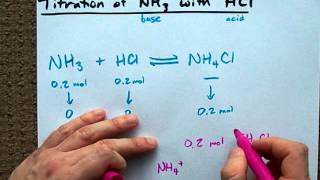Calculate the pH of a buffer prepared by mixing 300 cc of 0.3 M NH3 and 500 cc of 0.5 M NH4Cl . Kb for NH3 = 1.8 × 10^-5

Total Synthesis of (−)-Agelastatin A: The Application of a Sequential Sigmatropic Rearrangement | Organic Letters

Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage | PNAS

SOLVED: What is the most efficient way to increase the pH and buffer capacity of an ammonium (NH4+) /ammonia (NH3) buffer? dilute the buffer add ammonium chloride add ammonia add NaOH(aq) add

Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. - Abstract - Europe PMC

Pufferfish Rh glycoprotein-mediated uptake of the ammonia analog, [ 14... | Download Scientific Diagram

Seminaraufgaben - Übungen zur Vorlesung Anorganische Experimentalchemie (AC1 im WS) mit Lösungen. - Studocu

Thermo Scientific™ Orion™ ISE-Kalibrierstandards 0,1 M NH4Cl; 475 ml; CAS:(12125-02-9) Thermo Scientific™ Orion™ ISE-Kalibrierstandards | Fisher Scientific